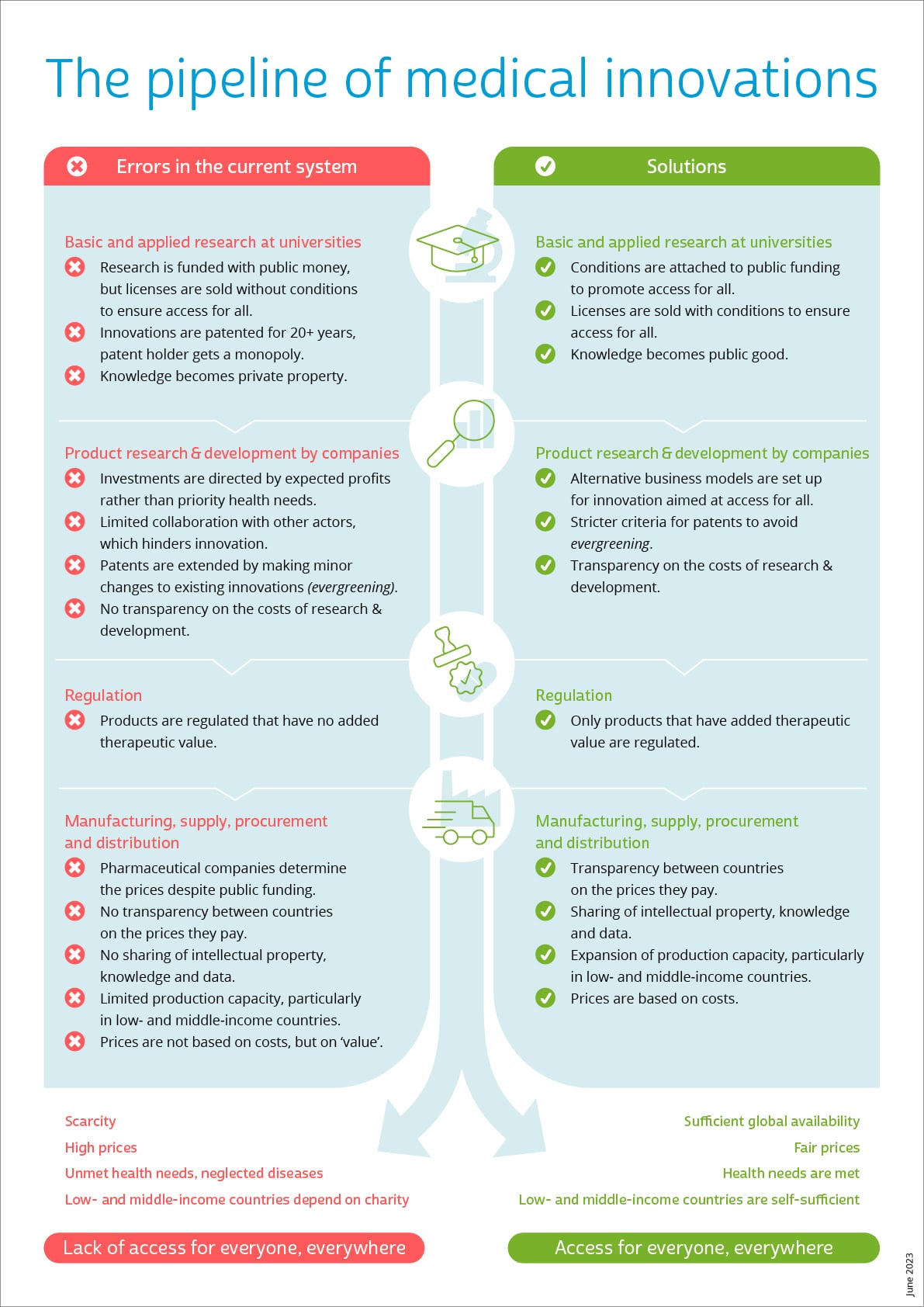
A fair system for medicine development
This visual illustrates the pipeline of medical product development, such as medicines and vaccines. It starts with basic and applied research at universities, and moves through research & development to regulation and finally to manufacturing and procurement.
All phases of pharmaceutical product development currently contain systemic flaws that prevent fair access to and affordability of medicines. New medicines are getting more expensive and more expensive, without pharmaceutical companies making it clear why.
We are convinced that the systemic errors can be fixed. Read below the visual what we think is needed for that.
If policymakers take equitable access to medicines as a starting point, the pipeline could look like this:
- At the basic and applied research stage, knowledge does not become private property for commercial purposes, but a global common good in the service of public health.
- When medical innovations that are developed with taxpayers’ money are sold to pharmaceutical companies, clear conditions are attached. For example, agreements on the prices, and on the sharing of knowledge and intellectual property, so that public health is placed above commercial interests.
- During the research & development phase, pharmaceutical companies are transparent about the costs they make in this phase. In addition, the sector experiments with business models that are not, or less, commercial, but rather fully focused on the public interest of health and access for all.
- For the medicines evaluation and approval phase, government agencies set rules to ensure that only products that actually have added therapeutic value reach the market.
- In the final stage of production, supply, procurement and distribution, pharmaceutical companies share their knowledge to scale up production and encourage further innovation. Prices are set on the basis of actual costs plus an established fair profit.